*Disclaimer: This article was published in 2019 and reflects the information available at that time.
With the influenza season fast approaching, demand on pharmacist vaccination services is imminent. Vaccination and immunisation services are covered under the PDL Master Policy but pharmacists providing these services must have current qualifications for vaccination and first aid support. Proprietors can take the lead by ensuring all staff involved in supporting pharmacy-based vaccination services meet accreditation requirements.
Incident reports to PDL and complaints to the regulators highlight there are several aspects of influenza vaccine supply and administration that are more likely to lead to an incident being reported. Incidents mainly relate to age, injury and administration technique.
Age-related Incidents
The most common cause for reporting relates to the age of the client. These incidents can be divided into three sub-groups as follows:
- Prescriptions for children
- Clients 65 years and over
- Clients vaccinated in the pharmacy who do not meet the State-legislated minimum age
The most common of the age-related incidents involve influenza vaccine prescriptions for children. Often these prescriptions are written generically for influenza vaccine. If the age of the patient i.e. the child, is not identified then there is a risk that the vaccine supplied is not approved for the child’s age.
Understandably, parents may be upset when informed the child has received a vaccine that is not approved for their child’s age. This may see a formal complaint being lodged with the regulator, to which the pharmacist must respond. It is important for pharmacists to provide a notification of incident report to PDL no matter how inconsequential the situation may seem at the time. Members are provided with immediate and personalised support through the reporting, regulatory and legal processes which may arise.
To limit the risk of an incident occurring, pharmacists and assistants receiving or processing prescriptions for influenza vaccines, must confirm the age of ALL patients. Furthermore, identifying vaccines stocked by the pharmacy with the approved age ranges, by way of a note or laminate for example, can prevent this oversight leading to an error.
The quadrivalent vaccines available in 2019 are as follows:
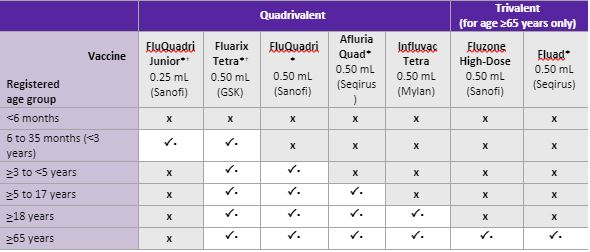
The second most common age-related report relates to patients 65+ years of age receiving pharmacist-administered quadrivalent influenza vaccines without the opportunity to consider or discuss the option of the high-dose trivalent vaccine. In some reports, the patient’s GP believed the patient should be vaccinated with the high-dose trivalent vaccine. This situation may lead to concern for the patient and conflict with the GP.
Refer to the ATAGI Clinical Advice 2019 and ensure any patient 65 years or over is advised of the availability of the trivalent vaccine and an appropriate discussion is undertaken to consider the options available to the patient. The quadrivalent may still be considered a suitable option in certain situations. Documentation of the discussion and decision is valuable in such cases.
The two vaccines available in 2019 for people 65 years or over are as follows:
- Fluad® is available to these clients under the NIP at no charge
- Fluzone High Dose® is NOT available under NIP, i.e. a non-PBS prescription item
Several cases have been identified whereby patients fall below the State-legislated minimum age for pharmacist vaccination. It is apparent that some of the patient consent proformas request a date of birth but not an age in years. PDL has made representations to providers of these forms regarding this aspect and the PSA consent form has been amended to incorporate this field. Update your pharmacy’s consent form to request the patient’s age in years.
There are also occasions when a parent may provide false information regarding the age of a child to facilitate vaccination in the pharmacy. If a pharmacist becomes aware after vaccination that the patient’s age has been misrepresented, then they are not responsible for this aspect. Refer to State legislation regarding recent changes to the minimum age for pharmacist-administered vaccination for influenza and other vaccines in several jurisdictions.
At the time of publication, the minimum age for vaccination is as follows:
| State | Minimum Age | Pharmacist-administered Vaccines |
| Qld | 16 | DTP (DTP-Polio if DTP unavailable), Influenza, MMR |
| NSW | 16 | DTP, Influenza, MMR |
| ACT | 16 | DTP, Influenza |
| Vic | 16 | DTP, Influenza (16 yrs & over following NIP guidelines i.e. Quad or Tri as per age, 16–64 yrs -non-NIP i.e. Quad only), MMR |
| Tas | 18 | Influenza |
| SA | 16 | DTP, DTP-Polio, Influenza, MMR (as per SA Health requirements) |
| NT | 16 | DTP, Influenza, MMR |
| WA | 18 | Influenza |
Injury
The most common injury reported to PDL with respect to pharmacist-administered vaccines is a condition known as SIRVA, i.e. Shoulder Injury Related to Vaccine Administration. This adverse outcome is known to all healthcare practitioners administering an injection into the deltoid muscle.
While this condition is usually resolved relatively quickly, the consequence for some patients can be significant and may include time off work. A small number of formal complaints to the regulators and/or claims for lost income have been seen with this injury.
Prompt action and appropriate referral is important if this adverse outcome is suspected. Pharmacists must administer vaccines as per their vaccination training and refer to the Immunisation Handbook.
Other areas of incident
Administration technique leading to leakage from the injection site, unsuitable or unprofessional area for vaccination and administration of expired vaccines are other areas of incident reported to PDL. Administration of expired vaccines is unlikely to occur unless stock from the previous year has not been removed or quarantined.
PDL members can call 1300 854 838 for advice and incident support from one of our Professional Officers. Supporting our pharmacist members 24/7.



